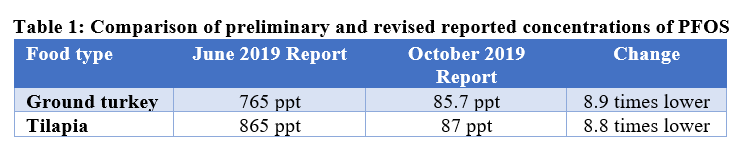
The Food and Drug Administration (FDA) recently released revised lab results from testing for 16 PFAS in food. Initial results of the testing were announced last June and gained wide attention because the levels of PFAS in certain foods were quite high. Surprisingly, the revised lab results show significantly fewer detections and, in the case of ground turkey and tilapia, concentrations of PFOS that are almost nine times lower than the values initially reported in June. In addition to the revised lab results, the agency also released a validated method for analyzing food for the substances and updated its PFAS webpage.
We were glad to see FDA’s ongoing work on PFAS and have already heard from commercial laboratories who are considering using the validated method as a potential new service to offer their customers. In analyzing the documentation that FDA provided,[1] we have concerns about the agency’s criteria to determine whether a sample had detectable levels of a PFAS. It appears unnecessarily restrictive and effectively underestimates the public’s exposure to PFAS. We are planning to meet with the agency to better understand their rationale for the criteria selection and its implications.
Let’s start with the positive news:
- Analytical method: The agency released a detailed description of its method to analyze bread, lettuce, milk and fish for 16 PFAS. We understand that the agency plans to expand the method to include more foods and more PFAS. This method is important since it provides a tool that stakeholders can use to test food for these chemicals with confidence. Given the concerns with PFAS contamination in food, we anticipate that commercial laboratories will begin offering testing services using the method – an important step to advance understanding of the issue.[2]
- False positive caused by chocolate: The agency explained that the initial results of 17,640 parts per trillion (ppt) in chocolate cake with icing and 154 ppt in low-fat chocolate milk for a PFAS known as PFPeA were false positives. The analytical method includes steps to avoid this problem going forward. In our June 2019 blog, we highlighted these results as surprising. We are pleased to see the agency has identified and fixed the problem with the method.
- Much less PFOS in meat and seafood than previously reported: FDA’s revised results show lower levels of PFOS in ground turkey and tilapia compared to initial values as follows:
In response to our request to better understand these marked differences, FDA told us that there was a calculation error in the original report related to dilution of the samples. This would apply to all of the meat, poultry, and seafood samples including the eight others that initially had PFOS concentrations between 134 and 676 ppt. We are pleased to see the lower numbers because the levels initially reported raised significant questions about the risk to consumers. While we know FDA strives to eliminate mistakes, they are part of life. The key is to correct them when they are found and clearly explain to its constituents the reason for the change.
FDA’s troubling method detection limit for measuring PFAS
The most dramatic change in the reported revised lab results is with the samples collected in North Carolina[3] and New Mexico[4] where 104 of the 182 (57%) detections reported in June 2019 were replaced with “<MDL” (less than the Method Detection Limit) in the new tables. They were initially reported as below the “Lower Limit of Quantification” with no further definition of the term or the value established for each food.
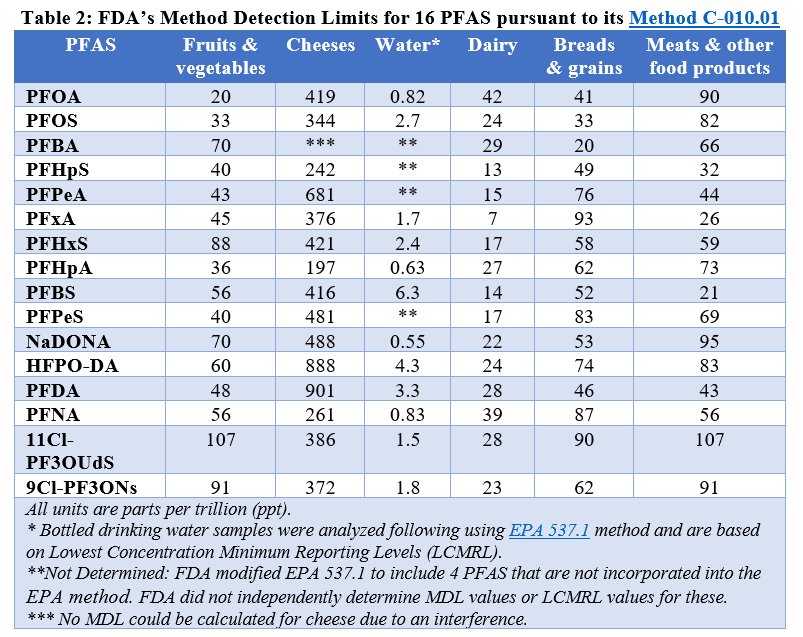
In the revised results page, the MDL, “is defined as the minimum concentration of a substance that can be measured and reported with 99% confidence that the analyte concentration is greater than zero.” In other words, it is the limit of detection of the analytical method for each PFAS in each type of food. FDA explains that this is the definition used in the Environmental Protection Agency’s (EPA) wastewater and sewage sludge permitting guidelines. These guidelines are intended to be used for regulatory compliance purposes. See Table 2 (at end of the blog) for the FDA’s MDLs for the 16 PFAS in six different food types (including bottled water).
In reviewing the agency’s documentation and past practices, we don’t fully understand FDA’s rationale for choosing EPA’s regulatory guidelines for wastewater and sewage sludge and why the agency concluded that they were the most appropriate criteria to assess the public’s exposure to PFAS in food. EPA’s guidelines are not mentioned in FDA’s Guidelines for the Validation of Chemical Methods, which the agency used to validate its PFAS analytical method. We have concerns that the application of such a restrictive criteria results in a MDL value reported as “non-detectable” even though the samples are highly likely to have detectable levels using more appropriate criteria such as those previously used by FDA.[5] As a result, the method is likely to underestimate food contamination and human exposure. We think it best to avoid this considering that PFAS have been associated with a host of health effects at incredibly low levels of exposure and some can bioaccumulate in the body.
We are planning to meet with the agency to better understand their rationale for the criteria selection and its implications.
Summary
A validated method to test food for PFAS is an important step forward. We anticipate that FDA and commercial laboratories will use it to evaluate more foods for more PFAS in the future. The method also serves as a foundation for a larger effort to assess public exposure to PFAS. Therefore, it is important, especially at this early stage in the effort, to have an MDL that does not screen out concentrations that are detectable by using overly restrictive criteria. We look forward to discussing those concerns with agency staff soon.
[1] We learned of the changes when FDA sent us an email responding to our July 30 Freedom of Information Act (FOIA) request. In that request, which we described in an August 3 blog, we asked for documents that explained how FDA concluded, based on its June preliminary results, that PFAS in food are safe.
[2] The agency described the method as validated. Normally, validating methods involves comparisons among multiple laboratories. For this method, the agency’s own lab did the validation using the Single Lab Validation Level described in its “Guidelines for the Validation of Chemical Methods for the FDA FVM Program 2nd Ed.” The link to the guideline, however was incorrect taking the reader to a validation of methods for the detection of microbial pathogens and not chemical contaminants. The proper link should be https://www.fda.gov/media/81810/download.
[3] In the North Carolina study of produce bought at farmer markets near a PFAS production plant in Fayetteville, the number of PFAS detections dropped from 108 to 36. See EDF’s notes of June 2019 results showing the changes.
[4] In the New Mexico study of dairy products collected from two farms near an air force base near Clovis, the number of PFAS detections dropped from 74 to 42. See EDF’s notes of June 2019 results showing the changes.
[5] FDA used a 95% confidence level for its 2015 validation method to measure five heavy metals in food for its Total Diet Study
By Tom Neltner, J.D., Chemicals Policy Director and Maricel Maffini, Ph.D., Consultant
Enviroshop is maintained by dedicated NetSys Interactive Inc. owners & employees who generously contribute their time to maintenance & editing, web design, custom programming, & website hosting for Enviroshop.