I’ve now examined dozens of papers and reports that EPA toxics nominee Michael Dourson and his firm, Toxicology Excellence for Risk Assessment (TERA), have published on chemicals over the past 15-20 years. A remarkably consistent pattern of how Dourson conducts his paid work for the chemical and pesticide industries emerges from this examination. I’ll use one example below to illustrate, but most or all of the steps I’ll describe have been followed over and over again.
The example I’ll use relates to two herbicides, alachlor and acetochlor (collectively known as acetanilides), widely u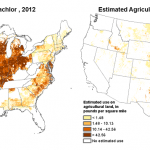 sed in huge volumes especially in the Midwest. The US Geological Survey reported that in 2015 about 2 million pounds of alachlor and more than 40 million pounds of acetochlor were used in agriculture annually. The USGS map images included here (click to enlarge) show where these substances are used, based on 2012 data.
sed in huge volumes especially in the Midwest. The US Geological Survey reported that in 2015 about 2 million pounds of alachlor and more than 40 million pounds of acetochlor were used in agriculture annually. The USGS map images included here (click to enlarge) show where these substances are used, based on 2012 data.
Dourson’s work specifically addressed the degradation products of alachlor and acetochlor, which are frequently detected in ground and surface waters. Except as otherwise noted below, the specifics I describe are recorded in documents posted on TERA’s webpage for this activity.
STEP 1: The process typically starts with a company or industry that has a problem or a decision it wants to influence, e.g.: a chemical has been spilled or is showing up in air or water monitoring; a facility permit is being reviewed; a government agency is doing a risk review of a chemical or updating a standard. In this case, Dow AgroSciences and Monsanto, makers of the acetanilides, were facing growing scrutiny as the herbicides’ degradation products were being routinely detected in ground and surface water samples and regulators in states like Minnesota were reviewing applicable water standards.
STEP 2: The affected company or industry group contracts with TERA to convene an “expert” panel or workshop or conduct a peer review of a government or industry assessment, research plan or other document. TERA is hired to convene and manage the panel or peer review. In this case, Dow AgroSciences and Monsanto hired TERA to run a workshop involving an “expert” panel that TERA was also to select.
STEP 3: TERA appoints its own founder and President, Michael Dourson, to the panel, almost always as Chair of the panel. This is a highly questionable practice: While the selection of panels and peer reviewers is sometimes contracted out to “third parties,” the procedures used are designed to keep the entity identifying experts and managing panels and associated meetings at arm’s length from the experts themselves. TERA makes no such effort: In the acetanilides case, as in the great majority of other TERA cases, employees of Dourson’s own company appointed him (their boss) to chair the “expert” panel.
STEP 4: TERA clears Dourson of any conflict of interest in his participation on the panel. That is, employees of Dourson’s own company are the sole determiners as to whether or not their boss has a conflict of interest in the matter at hand. Highly irregular, to say the least, an approach that presents its own conflicts of interest. In the acetanilides case, TERA cleared Dourson to serve on the panel even though TERA had recently contracted with both Dow AgroSciences and Monsanto to “provide technical review on projects.” This is not an isolated incident: In numerous other cases, TERA or Dourson himself had recently worked for the very same company or industry group paying TERA to convene a panel or conduct a review in which Dourson participated, typically as Chair.
STEP 5: Based on the workshop or review, Dourson and his colleagues write a paper for publication, sometimes involving other workshop or panel participants. In the acetanilides case, the first 5 of the 9 authors on the paper (including Dourson) were TERA employees.
STEP 6: The paper is typically published in the industry’s go-to journal, Regulatory Toxicology and Pharmacology. I have blogged earlier about the large fraction of Dourson’s papers – well over half – published in this one journal, which has a longstanding reputation of being the go-to journal for both tobacco and chemical industry-friendly paper publishing. The journal has been the subject of numerous exposés over the past 15 years regarding its close ties to the chemical and tobacco industries. True to form, in this case, Dourson’s paper was published in Regulatory Toxicology and Pharmacology. It recommended water quality standards for acetanilide degradation products many times less protective than those in place in Wisconsin and Minnesota.
Not all of these steps have occurred with every chemical. Dourson’s work on the likely carcinogen 1,4,-dioxane, for example, paid for by PPG Industries, doesn’t appear to have relied on an intervening workshop or “expert” panel for cover, and instead went straight to publication of a paper, once again in Regulatory Toxicology and Pharmacology. Not surprisingly, here too he argued for a far less health-protective standard, in this case about 1000-fold weaker than EPA’s level indicating an increased cancer risk. It’s worth noting that state agencies in Michigan and New Jersey reviewed Dourson’s work on this chemical and found it sorely lacking on scientific grounds.
It is not only Dourson’s deep conflicts of interest that lead us to oppose his nomination, but also his questionable science and incessant claims of independence, when in fact his whole step-by-step enterprise has been set up to bend the science in support of the interests of his corporate clients.
Richard Denison, Ph.D., is a Lead Senior Scientist.
[Use this link to see all of our posts on Dourson.]
Enviroshop is maintained by dedicated NetSys Interactive Inc. owners & employees who generously contribute their time to maintenance & editing, web design, custom programming, & website hosting for Enviroshop.
